Growth of BiFeO3 thin films by chemical solution deposition: the role of electrodes
Abstract
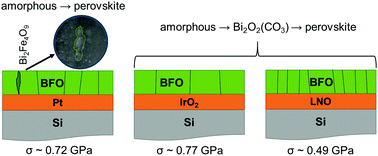
BiFeO3 (BFO) thin films were grown by chemical solution deposition on a range of electrodes to determine their role in controlling the phase formation and microstructure of the films. The crystallization on oxide electrodes followed the sequence: amorphous → Bi2O2(CO3) → perovskite, while those on Pt crystallized directly from the amorphous phase. IrO2 electrodes promoted perovskite phase formation at the lowest temperature and LaNiO3 additionally induced local epitaxial growth. All compositions exhibited fully coherent Fe-rich precipitates within the grain interior of the perovskite matrix, whereas the incoherent Bi2Fe4O9 second phase was also observed at the grain boundaries of BFO grown on Pt electrodes. The latter could be observed by X-ray diffraction as well as transmission electron microscopy (TEM) but coherent precipitates were only observed by TEM, principally evidenced by their Z contrast in annular dark field images. These data have pronounced consequences for the extended use of BFO films under an applied field for actuator, sensor and memory applications.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...