A theoretical study of complexes formed between cations and curved aromatic systems: electrostatics does not always control cation–π interaction†
Abstract
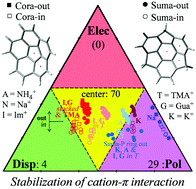
The present work studies the interaction of two extended curved π-systems (corannulene and sumanene) with various cations (sodium, potassium, ammonium, tetramethylammonium, guanidinium and imidazolium). Polyatomic cations are models of groups found in important biomolecules in which cation–π interaction plays a fundamental role. The results indicate an important size effect: with extended π systems and cations of the size of potassium and larger, dispersion is much more important than has been generally recognized for cation–π interactions. In most of the systems studied here, the stability of the cation–π complexes is the result of a balanced combination of electrostatic, induction and dispersion contributions. None of the systems studied here owes its stability to the electrostatic interaction more than 42%. Induction dominates stabilization in complexes with sodium, and in some of the potassium and ammonium complexes. In complexes with large cations and with flat cations dispersion is the major stabilizing contribution and can provide more than 50% of the stabilization energy. This implies that theoretical studies of the cation–π interaction involving large or even medium-size fragments require a level of calculation capable of properly modelling dispersion. The separation between the cation and the π system is another important factor to take into account, especially when the fragments of the cation–π complex are bound (for example, to a protein backbone) and cannot interact at the most favourable distance.


 Please wait while we load your content...
Please wait while we load your content...