Does H4SO5 exist?†
Abstract
The possible existence of H4SO5 in aqueous sulfuric acid is analyzed in detail. For bare H4SO5, the computed free energy barrier for the exergonic transformation of H4SO5 into the H2SO4⋯H2O complex is only 3.8 kcal mol−1. The presence of water or sulfuric acid catalyzes the dehydration to such an extent that it becomes almost a barrierless process. In the gas phase, dehydration of H4SO5 is an autocatalytic reaction as the water molecule produced by the decomposition of one H4SO5 molecule induces further dissociation. Thus, in solution, the surrounding water molecules make the para-sulfuric acid a very vulnerable species to exist. The simulated Raman spectra also corroborate the absence of H4SO5 in solution.
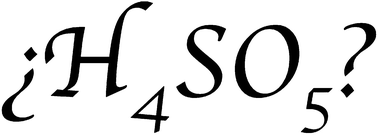


 Please wait while we load your content...
Please wait while we load your content...