Phase diagram of water–methane by first-principles thermodynamics: discovery of MH-IV and MH-V hydrates†
Abstract
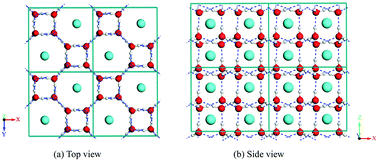
Searching novel gas hydrates is an enduring topic of scientific investigations, owing to its outstanding implications on planetology, the origin of life and the exploitation of energy resources. Taking the methane–water system as a representative, we disclose two new dense methane hydrate phases (MH-IV and MH-V) using the Monte-Carlo packing algorithm and density-functional theory (DFT) optimization. Both of these methane clathrates with (CH4)(H2O)4 stoichiometry can be regarded as filled ices, since their hydrogen bond networks are closely related to that of ice i and ice XI, respectively. In particular, the former ice i network is observed for the first time in all gas hydrates. A new chemical composition phase diagram of methane hydrate is constructed. Our newly identified methane hydrate IV emerges in the transition zone for a water–methane ratio between 2 : 1 and 5.75 : 1. It suggests that our MH-IV phase can be stabilized without external pressure, which is superior to previous reported filled ices to apply to energy storage. These findings attest to the importance of composition effects on the packing mechanism of gas hydrate, and provide new perspectives for understanding the physicochemical and geophysical processes in the giant planets of the solar system.


 Please wait while we load your content...
Please wait while we load your content...