Comparison of fast electron transfer kinetics at platinum, gold, glassy carbon and diamond electrodes using Fourier-transformed AC voltammetry and scanning electrochemical microscopy†
Abstract
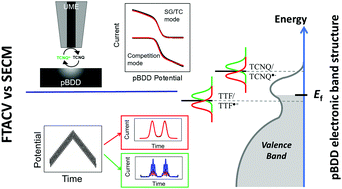
Heterogeneous electron transfer (ET) processes at electrode/electrolyte interfaces are of fundamental and applied importance and are extensively studied by a range of electrochemical techniques, all of which have various attributes but also limitations. The present study focuses on the one-electron oxidation of tetrathiafulvalene (TTF) and reduction of tetracyanoquinodimethane (TCNQ) in acetonitrile solution by two powerful electrochemical techniques: Fourier-transformed large amplitude alternating current voltammetry (FTACV); and scanning electrochemical microscopy (SECM), both of which are supported by detailed theoretical models. At conventional Pt, Au and glassy carbon (GC) electrode materials, the apparent (overall) charge transfer kinetic values determined by FTACV give standard ET rate constants, k0FTACV, that are fast and close to the reversible limit. They are in good agreement with highly localised k0SECM measurements determined by SECM under conditions of high mass transport rates. In contrast, the impact of both the complex heterogeneous surface of polycrystalline boron doped diamond (pBDD) and degenerate p-type doping results in a range of k0SECM values across the electrode surface compared to the overall k0FTACV measured for both processes studied. Moreover, the reduced availability of charge carriers at the electrode surface, at each energy state, compared to a metal, which decreases as the potential becomes more negative, results in lower k0 values at pBDD than Pt, Au and GC. The measurement technique also has an influence: SECM measurements are made at much higher local current density than FTACV, and for TCNQ/TCNQ˙−, which has the more negative formal potential, limited charge carrier availability results in k0FTACV > k0SECM, with unusual apparent charge transfer coefficients and voltammetric waveshapes from SECM. These data thus highlight the importance of understanding the influence of the measurement technique and further demonstrate how ET kinetics at pBDD differ from conventional electrodes, in this case for processes in an organic solvent, which has received much less attention compared to aqueous systems for studies with pBDD.
- This article is part of the themed collections: 2017 PCCP HOT Articles and RACI100: Celebrating Australian Chemistry


 Please wait while we load your content...
Please wait while we load your content...