A global coupled cluster potential energy surface for HCl + OH ↔ Cl + H2O
Abstract
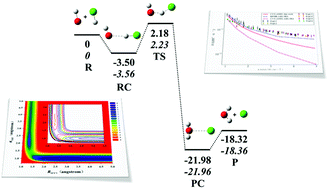
A new and more accurate full-dimensional global potential energy surface (PES) for the ground electronic state of the ClH2O system is developed by fitting 15 777 points obtained using an explicitly correlated unrestricted coupled-cluster method with single, double, and perturbative triple excitations (UCCSD(T)-F12b). The fitting is carried out using the permutation invariant polynomial-neural network (PIP-NN) method and has an error of 6.9 meV. The new PES has a slightly lower barrier for the atmospherically important HCl + OH → Cl + H2O reaction than the previous PES based on multi-reference configuration interaction (MRCI) calculations. As a result, it should provide a better characterization of the kinetics. Quantum dynamical calculations of reaction probabilities for both the forward and reverse reactions are performed on this new PES and compared with those on the MRCI PES. They reveal notable differences, resulting apparently from subtle differences in the PESs.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...