Fluorescence correlation spectroscopy study of the complexation of DNA hybrids, IgG antibody, and a chimeric protein of IgG-binding ZZ domains fused with a carbohydrate binding module†
Abstract
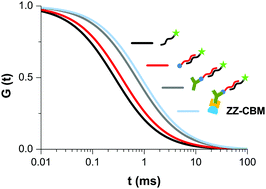
Fluorescence correlation spectroscopy (FCS) was used to characterize the molecular interactions between the four components of a DNA recognition system. A fluorescent DNA probe was used to assess: (i) the hybridization with a complementary biotin-labeled target, (ii) the complexation of the resulting hybrid and an anti-biotin antibody, and (iii) the binding of the latter complex to a ZZ-CBM fusion protein that combines small synthetic IgG Fc-binding Z domains with a carbohydrate binding module (CBM). These binding interactions were monitored by exposing the fluorescent DNA probe to different amounts and combinations of the other molecules in solution. Through the analysis of FCS autocorrelation curves, an association constant (Ka) of 2.9 × 107 M−1 was estimated for DNA·DNA hybridization, and the presence of (non-) complementary target DNA in solution could be discriminated. The specific capture of biotinylated DNA hybrids by anti-biotin IgG was verified, with an apparent Ka of 2.5 × 106 M−1. The increment in the diffusion time measured when the DNA·DNA:antibody complexes were in contact with the ZZ-CBM fusion protein suggested that the binding occurs at a stoichiometric ratio of DNA/antibody complex to fusion larger than 1 : 1. The FCS-derived information obtained is useful to gain insight into molecular interactions involved in diagnostic assays.


 Please wait while we load your content...
Please wait while we load your content...