Further insights into the kinetics of thermal decomposition during continuous cooling
Abstract
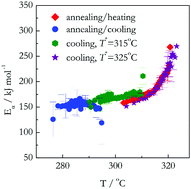
Following the previous work (Phys. Chem. Chem. Phys., 2016, 18, 32021), this study continues to investigate the intriguing phenomenon of thermal decomposition during continuous cooling. The phenomenon can be detected and its kinetics can be measured by means of thermogravimetric analysis (TGA). The kinetics of the thermal decomposition of ammonium nitrate (NH4NO3), nickel oxalate (NiC2O4), and lithium sulfate monohydrate (Li2SO4·H2O) have been measured upon heating and cooling and analyzed by means of the isoconversional methodology. The results have confirmed the hypothesis that the respective kinetics should be similar for single-step processes (NH4NO3 decomposition) but different for multi-step ones (NiC2O4 decomposition and Li2SO4·H2O dehydration). It has been discovered that the differences in the kinetics can be either quantitative or qualitative. Physical insights into the nature of the differences have been proposed.


 Please wait while we load your content...
Please wait while we load your content...