Extended timescale 2D IR probes of proteins: p-cyanoselenophenylalanine†
Abstract
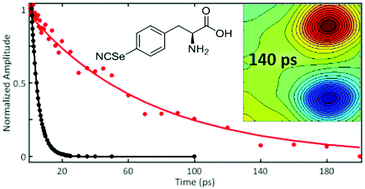
The importance of dynamics to the function of proteins is well appreciated, but the difficulty in their measurement impedes investigation into their precise role(s). 2D IR spectroscopy is a developing approach for the study of dynamics and has motivated efforts to develop spectrally resolved IR probe groups that enable its application for measuring the dynamics at specific sites in a protein. A challenge with this approach is that the timescales accessible are limited by the vibrational lifetimes of the probes. Toward development of better probes for 2D IR spectroscopy of protein dynamics, we report the characterization of p-cyano-seleno-phenylalanine (CNSePhe), a derivative of the well established IR probe p-cyano-phenylalanine (CNPhe), by FT IR, pump–probe, and 2D IR spectroscopy. The incorporation of the heavy Se atom decouples the CN vibration from the rest in the molecule. Although this leads to a reduction of the transition dipole strength, and thus a reduction in signal intensity, it also dramatically increases the vibrational lifetime, enabling collection of 2D IR spectra for analysis of molecular dynamics on much longer timescales. Interestingly, we also find that the lifetime for CNSePhe shows increased sensitivity to the presence of hydrogen bonding interactions with the CN, suggesting that the probe should be useful for interpretation of CN spectra and possibly for the study of solvation.


 Please wait while we load your content...
Please wait while we load your content...