Phonon bottleneck and long-lived excited states in π-conjugated pyrene hoop†
Abstract
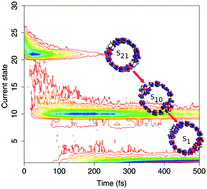
In the last decade, recent synthetic advances have launched carbon-based π-conjugated hoops to the forefront of theoretical and experimental investigation not only for their potential use as bottom-up templates for carbon nanotube (CNT) growth, but also for the interesting excitonic effects arising from the cyclic geometry, unique π-system orientation, and unusual electronic interactions and couplings. In particular, cyclic materials based on pyrene, a common component in organic electronics, are popular candidates for the future design of π-conjugated nanorings for optoelectronic applications. Understanding the photophysical response in cyclic oligopyrenes can be achieved using non-adiabatic excited state molecular dynamics (NA-ESMD). Through NA-ESMD modeling, we reveal details of the nonradiative relaxation processes in the circular pyrene tetramer [4]cyclo-2,7-pyrenylene ([4]CPY) where we find that the strong non-adiabatic coupling combined with the dense manifold of excited states creates an internal conversion mechanism dominated by ultrafast sequential quantum transitions. However, we observe two long-lived electronic excited states that introduce a phonon bottleneck in the electronic relaxation process. In fact, the timescale for the electronic relaxation is almost exclusively dominated by the lifetimes of the long-lived states. We find that the states associated with the phonon bottleneck are separated from lower energy states by large energy gaps and are characterized by localization on a single pyrene unit resulting in a spatial mismatch with strongly delocalized neighboring states.


 Please wait while we load your content...
Please wait while we load your content...