Can there be a multi-bond between noble gas and metal? A theoretical study of F2XeMoF2†
Abstract
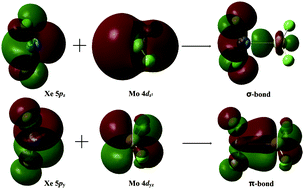
A new noble gas compound containing a Xe–Mo double bond, F2XeMoF2, was theoretically constructed and studied based on DFT and ab initio calculations. The CCSD(T)-calculated Xe–Mo bond length of 2.518 Å was comparable to the standard value of 2.56 Å. The bonding energy (32.3 kcal mol−1) was even higher than that of the Xe–Au bond in the well-known XeAuF complex (24.1 kcal mol−1). The result of natural bond orbital (NBO) analysis indicates that there is a σ-bond and a π-bond between the Xe and Mo atoms in F2XeMoF2. The properties of the Xe–Mo double bond were also analyzed with the atoms in molecules (AIM) approach and natural resonance theory (NRT).


 Please wait while we load your content...
Please wait while we load your content...