Analysis of charging-induced structural damage in electrochemical systems
Abstract
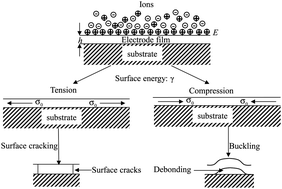
Electrochemical charging of an electrochemical system results in Maxwell stress and a variation in the surface energy of the electrode. The charging-induced variation in the surface energy of the electrode and Maxwell stress introduce mechanical deformation and create stresses in the electrode. To relax the strain energy stored in the electrode, surface cracking and buckling can occur. Under the condition that the charging-induced change in the surface energy is the dominant factor controlling the in-plane deformation of a planar electrode, i.e. the bonding between the adsorbate and the substrate is weak, the variation in the surface energy of the electrode can be described by the Lippmann equation. Using the Lippmann equation, both the surface charge density on the electrode and the surface energy of the electrode are formulated as a function of the electrode potential. Analytical relations between the critical electrode potential and average damage size have been obtained for the charging-induced cracking and buckling in a planar, thin-film electrode. The results show that surface cracking will prevail over local buckling in accordance with experimental observations. Both critical electrode potentials are inversely proportional to the square root of the magnitude of the differential capacity of the electrical double layer at the electrode potential of zero charge.


 Please wait while we load your content...
Please wait while we load your content...