Controlling the formation process and atomic structures of single pyrazine molecular junction by tuning the strength of the metal–molecule interaction
Abstract
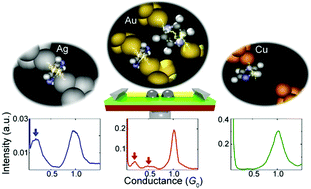
The formation process and atomic structures were investigated for single pyrazine molecular junctions sandwiched by three different Au, Ag, and Cu electrodes using a mechanically controllable break junction technique in ultrahigh vacuum conditions at 300 K. We demonstrated that the formation process of the single-molecule junction crucially depended on the choice of the metal electrodes. While single-molecule junction showing two distinct conductance states were found for the Au electrodes, only the single conductance state was evident for the Ag electrodes, and there was no junction formation for the Cu electrodes. These results suggested that metal–molecule interaction dominates the formation process and probability of the single-molecule junction. In addition to the metal–molecule interaction, temperature affected the formation process of the single-molecule junction. The single pyrazine molecular junction formed between Au electrodes exhibited significant temperature dependence where the junction-formation probability was about 8% at 300 K, while there was no junction-formation at 100 K. Instead of the junction formation, an Au atomic wire was formed at the low temperature. This study provides insight into the tuning of the junction-forming process for single-molecule junctions, which is needed to construct device structures on a single molecule scale.


 Please wait while we load your content...
Please wait while we load your content...