Stripping off hydrogens in imidazole triggered by the attachment of a single electron†
Abstract
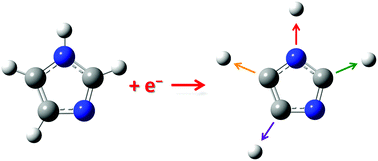
Imidazole [C3H4N2] is ubiquitous in nature as an important biological building block of amino acids, purine nucleobases or antibiotics. In the present study, dissociative electron attachment to imidazole shows low energy shape resonances at 1.52 and 2.29 eV leading to the most abundant dehydrogenated anion [imidazole − H]− through dehydrogenation at the N1 position. All the other anions formed exhibit core excited resonances observed dominantly at similar electron energies of ∼7 and 11 eV, suggesting an initial formation through two temporary negative ion states. Among these anions, multiple dehydrogenation reactions are observed resulting in the loss of 2 up to 4 hydrogens, thus, leading to a complete dehydrogenation of the imidazole molecule, an interesting prototype of complex unimolecular decay induced by the attachment of a single electron. Additionally, the quantum chemical calculations reveal that these multiple dehydrogenation reactions are responsible for the remarkable one electron-induced gas-phase chemistry leading to the opening of the ring. The formation of the observed anions is likely driven by the high positive electron affinity of cyanocarbon molecules supported by quantum chemical calculations. The formation of H− showed additional resonance at about 5 eV and dipolar dissociation above ∼14 eV.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...