Spin relaxation studies of Li+ ion dynamics in polymer gel electrolytes†
Abstract
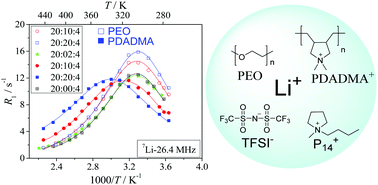
Two ternary polymer gel electrolyte systems are compared, containing either polyethylene oxide (PEO) or the poly-ionic liquid poly(diallyldimethylammonium) bis(trifluoromethyl sulfonyl)imide (PDADMA-TFSI). Both gel types are based on the ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethyl sulfonyl)imide (P14TFSI) and LiTFSI. We study the influence of the polymers on the local lithium ion dynamics at different polymer concentrations using 7Li spin–lattice relaxation data in dependence on frequency and temperature. In all cases the relaxation rates are well described by the Cole–Davidson motional model with Arrhenius dependence of the correlation time and a temperature dependent quadrupole coupling constant. For both polymers the correlation times are found to increase with polymer concentration. The activation energy of local motions slightly increases with increasing PEO concentration, and slightly decreases with increasing PDADMA-TFSI concentration. Thus the local Li+ motion is reduced by the presence of either polymer; however, the reduction is less effective in the PDADMA+ samples. We thus conclude that mechanical stabilization of a liquid electrolyte by a polymer can be achieved at a lower decrease of Li+ motion when a cationic polymer is used instead of PEO.


 Please wait while we load your content...
Please wait while we load your content...