Solid–liquid and liquid–solid transitions in metal nanoparticles
Abstract
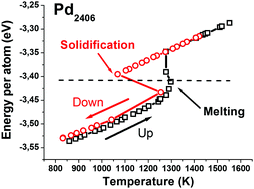
The melting and solidification temperatures of nanosystems may differ by several hundred Kelvin. To understand the origin of this difference, transitions in small metallic nanoparticles on the atomic scale were analyzed using molecular dynamics (MD). Palladium was used as a case study, which was then extended to a range of other elemental metals. It was argued that in realistic environments, such as gases at low pressure (of the order of 1 mbar), heat transfers allow the microcanonical thermal equilibrium evolution of the nanoparticles between successive collisions with gas atoms. This is shown to have no significant influence on the mechanism of melting, whereas in an isolated nanoparticle, solidification triggers a huge and rapid increase in temperature. A simple relationship between the melting and solidification temperatures was found, indicating that the magnitude of the latent heat of melting governs undercooling. Whereas melting occurs via heterogeneous nucleation, solidification displays characteristics of spinodal decomposition. Consistently, the melting temperature scales with the surface-to-volume ratio, whereas the solidification temperature displays no significant dependence on the particle size.


 Please wait while we load your content...
Please wait while we load your content...