Electronic non-adiabatic dynamics in enhanced ionization of isotopologues of hydrogen molecular ions from the exact factorization perspective
Abstract
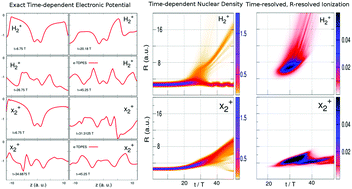
It was recently shown that the exact potential driving the electron's dynamics in enhanced ionization of H2+ can have large contributions arising from dynamic electron–nuclear correlation, going beyond what any Coulombic-based model can provide. This potential is defined via the exact factorization of the molecular wavefunction that allows the construction of a Schrödinger equation for the electronic system, in which the potential contains exactly the effect of coupling to the nuclear system and any external fields. Here we study enhanced ionization in isotopologues of H2+ in order to investigate the nuclear-mass-dependence of these terms for this process. We decompose the exact potential into components that naturally arise from the conditional wavefunction, and also into components arising from the marginal electronic wavefunction, and compare the performance of propagation on these different components as well as approximate potentials based on the quasi-static or Hartree approximation with the exact propagation. A quasiclassical analysis is presented to help analyse the structure of different non-Coulombic components of the potential driving the ionizing electron.


 Please wait while we load your content...
Please wait while we load your content...