Theoretical investigation of gas-phase molecular complex formation between 2-hydroxy thiophenol and a water molecule†
Abstract
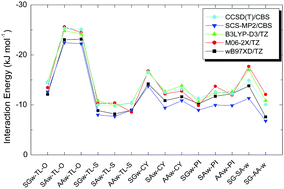
The torsional potential of OH and SH rotations in 2-hydroxy thiophenol is systematically studied using the MP2 ab initio method. The outcome of state-of-the-art calculations is used in the investigation of the structures and conformational preferences of 2-hydroxy thiophenol and aims at further interaction studies with a gas phase water molecule. SCS-MP2 and CCSD(T) complete basis set (CBS) limit interaction energies for these complexes are presented. The SCS-MP2/CBS limit is achieved using various two-point extrapolation methods with aug-cc-pVDZ and aug-cc-pVTZ basis sets. The CCSD(T) correction term is determined as the difference between CCSD(T) and SCS-MP2 interaction energies calculated using a smaller basis set. The effect of counterpoise correction on the extrapolation to the CBS limit is discussed. The performance of DFT based wB97XD, M06-2X and B3LYP-D3 functionals is tested against the benchmark energy from ab initio calculations. Hydrogen bond interactions are characterized by carrying out QTAIM, NCIPLOT, NBO and SAPT analyses.

 Please wait while we load your content...
Please wait while we load your content...