Chiral organocatalysts based on lipopeptide micelles for aldol reactions in water†
Abstract
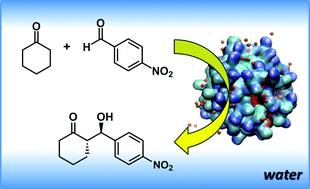
A comprehensive study of the self-assembly in water of a lipopeptide consisting of a sequence of L-proline, L-arginine and L-tryptophan with a hydrocarbon chain has been performed. Fluorescence assays were used to determine the critical aggregation concentration. In situ small-angle X-ray scattering (SAXS) and molecular dynamics simulations showed the presence of spherical micelles with diameters around 6 nm. In agreement with these results, cryo-TEM images showed globular aggregates with diameters ranging from ≈4 nm up to ≈9 nm. Furthermore, the lipopeptide catalytic activity has been tested for the direct aldol reaction between cyclohexanone and p-nitrobenzaldehyde, and we have observed that the self-association of the organocatalyst played a critical role in the enhanced activity. Water affects the selectivity, and poor results are obtained under neat reaction conditions. The location of the catalytic groups at the lipopetide/water solvent interface also endowed unusual selectivity in the catalyzed aldol reactions. Under optimized reaction conditions, high yields (up to >99%), good enantioselectivity (ee up to 85%) and high diastereoselectivity (ds up to 92 : 8) were obtained.
- This article is part of the themed collection: SBQ-RSC: Celebrating UK-Brazil collaborations


 Please wait while we load your content...
Please wait while we load your content...