Nanostructure, hydrogen bonding and rheology in choline chloride deep eutectic solvents as a function of the hydrogen bond donor
Abstract
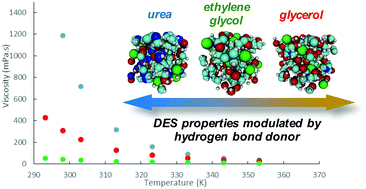
Deep eutectic solvents (DESs) are a mixture of a salt and a molecular hydrogen bond donor, which form a eutectic liquid with a depressed melting point. Quantum mechanical molecular dynamics (QM/MD) simulations have been used to probe the 1 : 2 choline chloride–urea (ChCl : U), choline chloride–ethylene glycol (ChCl : EG) and choline chloride–glycerol (ChCl : Gly) DESs. DES nanostructure and interactions between the ions is used to rationalise differences in DES eutectic point temperatures and viscosity. Simulations show that the structure of the bulk hydrogen bond donor is largely preserved for hydroxyl based hydrogen bond donors (ChCl:Gly and ChCl:EG), resulting in a smaller melting point depression. By contrast, ChCl:U exhibits a well-established hydrogen bond network between the salt and hydrogen bond donor, leading to a larger melting point depression. This extensive hydrogen bond network in ChCl:U also leads to substantially higher viscosity, compared to ChCl:EG and ChCl:Gly. Of the two hydroxyl based DESs, ChCl:Gly also exhibits a higher viscosity than ChCl:EG. This is attributed to the over-saturation of hydrogen bond donor groups in the ChCl:Gly bulk, which leads to more extensive hydrogen bond donor self-interaction and hence higher cohesive forces within the bulk liquid.


 Please wait while we load your content...
Please wait while we load your content...