High chloride content calcium silicate glasses†
Abstract
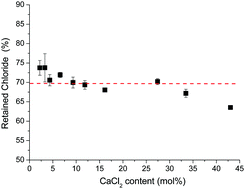
Chloride is known to volatilize from silicate glass melts and until now, only a limited number of studies on oxychloride silicate glasses have been reported. In this paper we have synthesized silicate glasses that retain large amounts of CaCl2. The CaCl2 has been added to the calcium metasilicate composition (CaO·SiO2). Glasses were produced via a melt quench route and an average of 70% of the chloride was retained after melting. Up to 31.6 mol% CaCl2 has been successfully incorporated into these silicate glasses without the occurrence of crystallization. 29Si MAS-NMR spectra showed the silicon being present mainly as a Q2 silicate species. This suggests that chloride formed Cl–Ca(n) species, rather than Si–Cl bonds. Upon increasing the CaCl2 content, the Tg reduced markedly from 782 °C to 370 °C. Glass density and glass crystallization temperature decreased linearly with an increase in the CaCl2 content. However, both linear regressions revealed a breakpoint at a CaCl2 content just below 20 mol%. This might be attributed to a significant change in the structure and is also correlated with the nature of the crystallizing phases formed upon heat treatment. The glasses with less than 19.2 mol% CaCl2 crystallized to wollastonite, whilst the compositions with CaCl2 content equal to or greater than 19.2 mol% are thought to crystallize to CaCl2. In practice, the crystallization of CaCl2 could not occur until the crystallization temperature fell below the melting point of CaCl2. The implications of the results along with the high chloride retention are discussed.


 Please wait while we load your content...
Please wait while we load your content...