Ionic conductivity and mixed-ion effect in mixed alkali metaphosphate glasses
Abstract
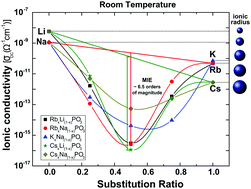
In this work, mixed alkali metaphosphate glasses based on K–Na, Rb–Na, Rb–Li, Cs–Na and Cs–Li combinations were studied by differential scanning calorimetry (DSC), complex impedance spectroscopy, and Raman spectroscopy. DSC analyses show that both the glass transition (Tg) and melting temperatures (Tm) exhibit a clear mixed-ion effect. The ionic conductivity shows a strong mixed-ion effect and decreases by more than six orders of magnitude at room temperature for Rb–Na or Cs–Li alkali pairs. This study confirms that the mixed-ion effect may be explained as a natural consequence of random ion mixing because ion transport is favoured between well-matched energy sites and is impeded due to the structural mismatch between neighbouring sites for dissimilar ions.


 Please wait while we load your content...
Please wait while we load your content...