The dynamic binding of cholesterol to the multiple sites of C99: as revealed by coarse-grained and all-atom simulations†
Abstract
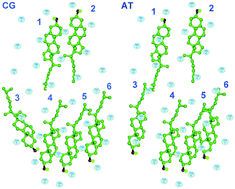
It is generally believed that the etiology of Alzheimer's disease (AD) is closely related to the amyloid-β polypeptides, produced from γ-secretase cleavage of C99. There is preliminary evidence that cholesterol directly activates γ-secretase cleavage of C99 through mechanisms that have not been understood so far. In this article, coarse-grained (CG) and all-atom (AT) simulations were employed to investigate the association between C99 and cholesterol, which is essential for our understanding of the role of cholesterol in the amyloidogenic pathway. Firstly, we find that both the N-terminus and the C-terminus of the C99 transmembrane domain (TMD) show interactions with cholesterol. Secondly, a multi-site dynamic cholesterol binding model was captured from the simulations, where 6 binding sites in the C99 TMD were presented. The analyses of the binding energies show that cholesterol prefers the site no. 1, 2, 4 and 5 over others. The most favorable binding energy of nearly −58.857 kJ mol−1 is from site 1, the repeat GxxxG motif. There are two pathways and two binding states of cholesterol binding to this site. Ser697 and Phe690 contribute most to the stabilization of the tightly binding state and the loosely binding state, respectively. The other binding sites described may also be potential drug targets. Thirdly, the residues GAVILMTKF, especially IVKF play a key role in this association. The C99 model appears to suggest a new mechanism for cholesterol binding. Finally, the multiple-site dynamic cholesterol binding model better explains the hypotheses that cholesterol promotes the amyloidogenic AβPP route. The GxxxA motif in the middle of the C99 transmembrane domain is completely exposed without cholesterol sheltering, which might help γ-secretase identify the cleavage sites and then promote γ-cleavage. Our results provide a detailed picture of dynamic cholesterol binding, which is crucial to our recognition of the potential influence of cholesterol on the C99 process and the etiology of AD.


 Please wait while we load your content...
Please wait while we load your content...