Mechanisms of absorption and desorption of CO2 by molten NaNO3-promoted MgO†
Abstract
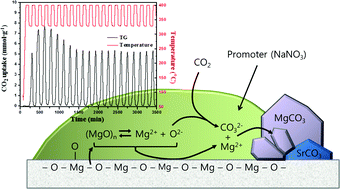
In order to realize carbon capture and sequestration (CCS), a technology proposed to circumvent the global warming problem while maintaining the present level of economic activity, the development of efficient carbon-capturing agents is of prime importance. In addition to the prevailing amine-based agents that operate at temperatures lower than 200 °C, agents that can operate at higher temperatures are being considered to reduce the cost of CCS. For the mid-temperature (200–500 °C) operation, alkali nitrate-promoted MgO is a promising candidate; whose detailed reaction mechanisms are not yet fully understood, however. In the present study, we have performed a comprehensive investigation on the mechanisms of CO2 absorption and desorption of NaNO3-promoted MgO. Highly efficient CO2 absorbents were obtained by decomposing Mg5(CO3)4(OH)2·4H2O with NaNO3 intimately mixed with it. Our collective data, including isothermal CO2 uptake curves, MgO solubility in molten NaNO3, and observations on the reaction of MgO wafers with CO2, indicate that the absorption takes place in the molten NaNO3 medium in which both CO2 and MgO are dissolved. MgCO3 is formed inside the molten promoter through the nucleation and growth steps. The decomposition of MgCO3 back to MgO, that is desorption of CO2, is also facilitated by molten NaNO3, which we attribute to the decreased relative stability of MgCO3 with respect to MgO when in contact with molten NaNO3. The relative affinity of molten nitrate to MgO and MgCO3 was estimated by measuring the ‘contact angles’ of nitrate on them. Implications of our findings for the real applications of alkali nitrate-promoted MgO absorbents with numerous repeated cycles of absorption and desorption of CO2 are discussed.


 Please wait while we load your content...
Please wait while we load your content...