Isotopic studies of the ammonia decomposition reaction using lithium imide catalyst†
Abstract
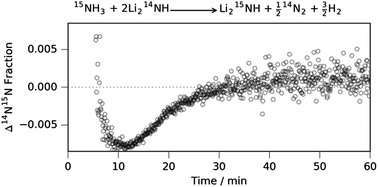
Ammonia decomposition using 15N labelled ammonia was performed over a lithium imide catalyst with mass spectrometry. The results show that all the nitrogen is released from the bulk of the lithium imide catalyst during the ammonia decomposition reaction, but that the decomposition itself occurs at the catalyst surface; they also indicate that lithium imide decomposes ammonia and does not merely act as a promoter to transition metal catalysts.


 Please wait while we load your content...
Please wait while we load your content...