A theoretical study of the potential energy surfaces for the double proton transfer reaction of model DNA base pairs
Abstract
The excited-state double proton transfer (ESDPT) mechanism in a model DNA base pair, 7-azaindole (7AI) dimer, has been debated over the years. Recently, Otero and coworkers concluded that the stepwise mechanism is not possible and the concerted mechanism dominates the dynamics (Chem. Sci., 2015, 6, 5762). In this work, the potential energy surfaces of the 7AI dimer in the ground state (S0) and the lowest energy excited singlet state (S1) were constructed. After vertical excitation to the S1 state, the single proton transfer can occur. The second proton transfer process in the stepwise mechanism is blocked by a high potential barrier (36.4 kcal mol−1), which is consistent with the result proposed by Otero and coworkers. However, the single proton transfer process is compatible with the concerted mechanism and we show that the single proton transfer process rather than the concerted mechanism dominates the dynamics. The concerted process is unfavorable in the S1 state compared with the barrierless single proton transfer process. In addition, the proton transfer process in the S0 state is revealed. The single proton transfer tautomer in the S1 state returns to the S0 state and transfers the second proton via a barrierless process. Finally, the double proton transfer tautomer in the S0 state can recover to the normal dimer through the reverse proton transfer reaction.
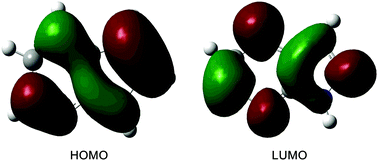


 Please wait while we load your content...
Please wait while we load your content...