New insights into the electroreduction of ethylene sulfite as an electrolyte additive for facilitating solid electrolyte interphase formation in lithium ion batteries†
Abstract
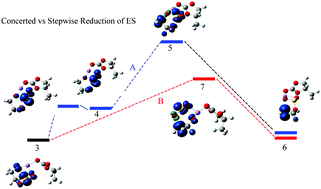
To help understand the solid electrolyte interphase (SEI) formation facilitated by electrolyte additives of lithium-ion batteries (LIBs) the supermolecular clusters [(ES)Li+(PC)m](PC)n (m = 1–2; n = 0, 6 and 9) were used to investigate the electroreductive decompositions of the electrolyte additive ethylene sulfite (ES) as well as the solvent propylene carbonate (PC) with density functional theory. The results show that ES can be reduced prior to PC, resulting in a reduction precursor that will then undergo a ring opening decomposition to yield a radical anion. A new concerted pathway (path B) was located for the ring opening of the reduced ES, which has a much lower energy barrier than the previously reported stepwise pathway (path A). The transition state for the ring opening of PC induced by the reduced ES (path C, indirect path) is closer to that of path A than path B in energy. The direct ring opening of the reduced PC (path D) has a lower energy barrier than paths A, B and C, yet it is less favorable than the latter paths in terms of thermodynamics (vertical electron affinity or reduction potential and dissociation energy). The overall rate constant including the initial reduction and the subsequent ring opening for path B is the largest among the four paths, followed by paths A > C > D, which further signifies the importance of the concerted new path in facilitating the SEI formation. The hybrid models, the supermolecular clusters augmented by a polarized continuum model, PCM-[(ES)Li+(PC)2](PC)n (n = 0, 6 and 9), were used to further estimate the reduction potential by taking into account both explicit and implicit solvent effects. The second solvation shell of Li+ in [(ES)Li+(PC)2](PC)n (n = 6 and 9) partially compensates the overestimation of solvent effects arising from the PCM for the naked (ES)Li+(PC)2, and the theoretical reduction potential of PCM-[(ES)Li+(PC)2](PC)6 (1.90–1.93 V) agrees very well with the experimental one (1.8–2.0 V).


 Please wait while we load your content...
Please wait while we load your content...