Photoinduced δ electron transfer in phenylene bridged Mo2 dimers†
Abstract
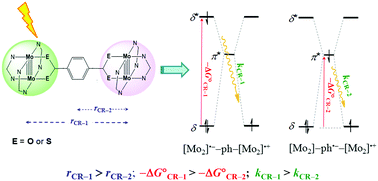
Photoinduced electron transfer (ET) in Mo2 dimers, [Mo2(DAniF)3]2(μ-E2C(ph)CE2) (DAniF = N,N′-di(p-anisyl)formamidinate, E = O or S), has been studied by femtosecond transient spectral techniques. Using a 355 nm laser pulse, the δ electrons on the Mo–Mo quadruple bonds are selectively excited and subsequent charge separation yields diradicals [Mo2]˙−–ph–[Mo2]˙+ and [Mo2]–ph˙−–[Mo2]˙+. The charge separation (CS) and recombination (CR) rates are derived by fitting the decay kinetic data of the excited states. Surprisingly, it is found that the CR rate constants (kCR-1, ∼1012 s−1) for [Mo2]˙−–ph–[Mo2]˙+ are larger than the data (kCR-2) for [Mo2]–ph˙−–[Mo2]˙+ by about one order of magnitude, although in the first case, the ET distance is doubled and the electronic coupling between the donor and acceptor is weaker. Optical analyses reveal that the free energy changes (ΔG°) for the two CR processes correspond to the δ → δ* and the metal (δ) to bridging ligand (π*) transition energies, respectively, and thus, the ET kinetics is dominated likely by the driving force (−ΔG°).

 Please wait while we load your content...
Please wait while we load your content...