Electrolyte-controlled discharge product distribution of Na–O2 batteries: a combined computational and experimental study†
Abstract
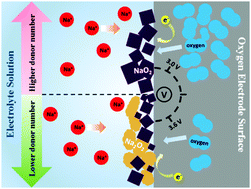
Tuning the composition of discharge products is an important strategy to reduce charge potential, suppress side reactions, and improve the reversibility of metal–oxygen batteries. In the present study, first-principles calculations and experimental confirmation were performed to unravel the influence of O2 pressure, particle size, and electrolyte on the composition of charge products in Na–O2 batteries. The electrolytes with medium and high donor numbers (>12.5) are favorable for the formation of sole NaO2, while those with low donor numbers (<12.5) may permit the formation of Na2O2 by disproportionation reactions. Our comparative experiments under different electrolytes confirmed the calculation prediction. Our calculations indicated that O2 pressure and particle size hardly affect discharge products. On the electrode, only one-electron-transfer electrochemical reaction to form NaO2 takes place, whereas two-electron-transfer electrochemical and chemical reactions to form Na2O2 and Na3O4 are prevented in thermodynamics. The present study explains why metastable NaO2 was identified as a sole discharge product in many experiments, while thermodynamically more stable Na2O2 was not observed. Therefore, to achieve low overpotential, a high-donor-number electrolyte should be applied in the discharge processes of Na–O2 batteries.


 Please wait while we load your content...
Please wait while we load your content...