Structures of protonated hydrogen sulfide clusters, H+(H2S)n, highlighting the nature of sulfur-centered intermolecular interactions†
Abstract
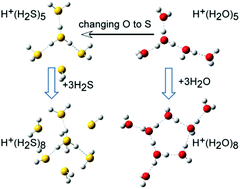
Unique intermolecular structures of protonated hydrogen sulfide clusters, H+(H2S)n, are revealed by infrared spectroscopy and ab initio calculations. The identified intermolecular structures are significantly different from those of the corresponding protonated water clusters, H+(H2O)n, in spite of the common hydrogen bond coordination ability between hydrogen sulfide and water. Protonated hydrogen sulfide clusters have the Eigen type ion core, H3S+, in the size range of n = 3–9. After the first hydrogen bonded shell formation is completed at n = 4, further solvation prefers a new shell bound by the charge–dipole interaction rather than the second hydrogen bonded shell. Thus, closely solvated structures, in which 7 molecules, at maximum, directly interact with the Eigen type ion core, are formed. The beginning of the second hydrogen bonded shell is found at n = 9. Competition among intermolecular interactions in H+(H2S)n is discussed.

 Please wait while we load your content...
Please wait while we load your content...