The deactivation mechanism of Pb on the Ce/TiO2 catalyst for the selective catalytic reduction of NOx with NH3: TPD and DRIFT studies†
Abstract
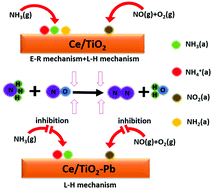
It was well recognized that Pb had a poisoning effect on a SCR catalyst. In this study, the deactivation mechanism of Pb on the Ce/TiO2 catalyst was investigated based on the characterization results of TPD and in situ DRIFT studies. It was found that the addition of Pb on the Ce/TiO2 catalyst not only inhibited the adsorption and activation of NH3 species, but also led to the decrease of the activity of adsorbed NH3 species in the SCR reaction. The adsorption of NOx species and the oxidation of NO by O2 over the Ce/TiO2 catalyst were also suppressed by the addition of Pb, while the reactivity of adsorbed NO2 species did not decrease. Moreover, the results revealed that the NH3-SCR reaction over the Ce/TiO2 catalyst followed both the E–R and L–H mechanisms, while the NH3-SCR reaction over Ce/TiO2-Pb was mainly controlled by the L–H mechanism. The contributions of the L–H mechanism to the SCR reactions over Ce/TiO2 and Ce/TiO2-Pb decreased with increasing reaction temperature. The deactivation of Ce/TiO2-Pb was mainly attributed to the suppressed NH3 adsorption and activation, accompanied by the inhibited NO oxidation and the decrease of Brønsted acid sites.


 Please wait while we load your content...
Please wait while we load your content...