Protonation of aqueous alanine by photoionization of water
Abstract
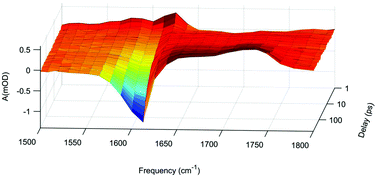
Hydronium ions produced by photolysis of water are used to study the protonation dynamics of alanine zwitterions in water. The measurements are done by UV-VIS and UV-IR femtosecond transient absorption spectroscopy on alanine in H2O and D2O. It is estimated that the reaction rate constant for the deuteration of alanine zwitterions is 4 × 1010 M−1 s−1, while the reverse process has a rate constant of 2 × 108 s−1. In addition to hydronium ions the photolysis of water yields hydrogen atoms and hydrated electrons together with hydroxyl radicals and hydroxyl ions. However, no other products resulting from reactions between aqueous alanine and the photolysis products of water are positively identified during the first 530 ps after the photolysis. Potential secondary reactions that are not observed experimentally are discussed and an upper limit is set for their yield where possible.


 Please wait while we load your content...
Please wait while we load your content...