Ultra-fast charge migration competes with proton transfer in the early chemistry of H2O˙+†
Abstract
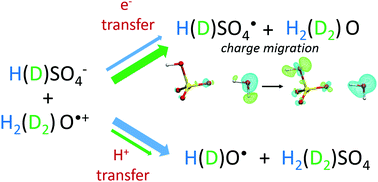
Oxidation by the ultra-short lived radical cation of water, H2O˙+, can potentially take place at the interface of water and numerous heterogeneous systems involved in radiation therapy, energy and environmental industries. The oxidation processes induced by H2O˙+ can be mimicked in highly concentrated solutions where the nearest neighbors of H2O˙+ may be molecules other than water. The reactivity of H2O˙+ and D2O˙+ is probed in hydrogenated and deuterated sulfuric acid solutions of various concentrations. The oxidized solute, sulfate radical, is observed at 7 ps and remarkably higher yields are found in deuterated solutions. The isotopic effects reveal the competition between two ultrafast reactions: proton transfer toward H2O (D2O) and electron transfer from HSO4− to H2O˙+ (D2O˙+). Density functional theory simulations decipher the electron transfer mechanism: it proceeds via sub-femtosecond charge migration and is not affected by isotopic substitution. This work definitively demonstrates why direct oxidation triggered by H2O˙+ can be competitive with proton transfer.


 Please wait while we load your content...
Please wait while we load your content...