Molecular aggregation of naphthalimide organic semiconductors assisted by amphiphilic and lipophilic interactions: a joint theoretical and experimental study†
Abstract
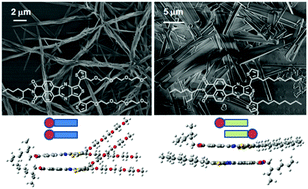
Amphiphilic and lipophilic donor–acceptor naphthalimide–oligothiophene assemblies exhibiting almost identical intramolecular properties, but differing in their intermolecular interactions, have been synthesized. Here we analyze the effect of replacing the normally used lipophilic alkyl chains with hydrophilic ones in directing molecular aggregation from an antiparallel to a parallel stacking. This different molecular packing of the amphiphilic, NIP-3TAmphi, and lipophilic, NIP-3TLipo, systems is assessed by electronic spectroscopies, scanning electronic microscopy and DFT quantum-chemical calculations. Theoretical calculations indicate that the presence of amphiphilic interactions promotes a face-to-face parallel arrangement of neighbor molecules, which induces improved electronic coupling and therefore enhances the charge transport ability and photoconducting properties of this type of materials. Time of flight and photoconducting measurements are used to determine the impact of the amphiphilic and lipophilic interactions on their possible performance in optoelectronic devices.


 Please wait while we load your content...
Please wait while we load your content...