Seeing the invisible plasma with transient phonons in cuprous oxide†
Abstract
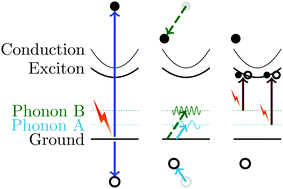
The emission of phonons from electron–hole plasma is the primary limit on the efficiency of photovoltaic devices operating above the bandgap. In cuprous oxide (Cu2O) there is no luminescence from electron–hole plasma. Therefore, we searched for optical phonons emitted by energetic charge carriers using phonon-to-exciton upconversion transitions. We found 14 meV phonons with a lifetime of 0.916 ± 0.008 ps and 79 meV phonons that are longer lived and overrepresented. It is surprising that the higher energy phonon has a longer lifetime.


 Please wait while we load your content...
Please wait while we load your content...