Controlled synthesis of carbon-supported Co catalysts from single-sites to nanoparticles: characterization of the structural transformation and investigation of their oxidation catalysis†
Abstract
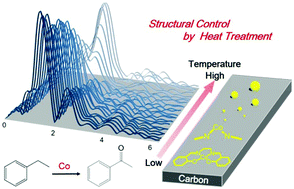
Realizing accurate control of catalytically active centers on solid surfaces is one of the most essential goals in the development of functionalized heterogeneous catalysts. Controlled synthesis of carbon-supported Co catalysts from single-site to nanoparticles can be successfully achieved by the structural transformation of the deposited Co(salen) complex precursor under heat treatment. The obtained structures were characterized using techniques such as XRD, in situ XAFS, and TEM. The first decomposition of the Co(salen) complex is initiated by the dissociation of Co–O–C bonds at around 250 °C, which produces isolated single-atom Co species while retaining the Co–N–C bonds even up to 400 °C. When the heat treatment temperature exceeds 450 °C, the second decomposition of the Co–N–C bonds occurs to form Co oxide nanoclusters followed by the growth of Co NPs upon further increase of the heat treatment temperature. The single-site catalyst is highly dispersed and electronically deficient owing to the interaction with the carbon support, and shows activity and selectivity for the oxidation of ethylbenzene, as compared to the inherent Co(salen) complex and nanoparticle catalysts.

 Please wait while we load your content...
Please wait while we load your content...