Conformational equilibrium and internal dynamics in the iso-propanol–water dimer†
Abstract
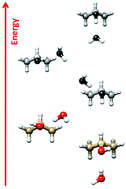
The molecular complex between iso-propanol and water has been investigated by Fourier transform microwave spectroscopy. Two distinct rotational spectra have been assigned, corresponding to two different isomers of the adduct. In both cases the water molecule acts as a proton donor to the alcoholic oxygen atom of iso-propanol in its gauche arrangement. The isomer in which the water molecule is oriented along the symmetry plane of the iso-propanol molecule (inner) is more stable than the second isomer, where the water is positioned outside the iso-propanol symmetry plane (outer). The rotational transitions of the inner isomer display a doubling, due to the two equivalent minima related to the internal rotation of the hydroxyl group (concerted with a rearrangement of the water unit). The tunneling splitting has been determined to be 25.16(8) GHz, corresponding to a B2 barrier of ∼440 cm−1.

 Please wait while we load your content...
Please wait while we load your content...