Substituent effects on the relaxation dynamics of furan, furfural and β-furfural: a combined theoretical and experimental approach†
Abstract
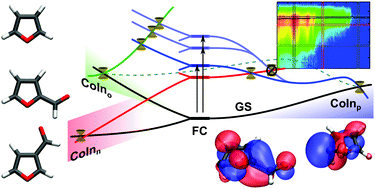
For the series furan, furfural and β-furfural we investigated the effect of substituents and their positioning on the photoinduced relaxation dynamics in a combined theoretical and experimental approach. Using time resolved photoelectron spectroscopy with a high intensity probe pulse, we can, for the first time, follow the whole deactivation process of furan through a two photon probe signal. Using the extended 2-electron 2-orbital model [Nenov et al., J. Chem. Phys., 2011, 135, 034304] we explain the formation of one central conical intersection and predict the influence of the aldehyde group of the derivatives on its geometry. This, as well as the relaxation mechanisms from photoexcitation to the final outcome was investigated using a variety of theoretical methods. Complete active space self consistent field was used for on-the-fly calculations while complete active space perturbation theory and coupled cluster theory were used to accurately describe critical configurations. Experiment and theory show the relaxation dynamics of furfural and β-furfural to be slowed down, and together they disclose an additional deactivation pathway, which is attributed to the nO lonepair state introduced with the aldehyde group.


 Please wait while we load your content...
Please wait while we load your content...