A full dimensional potential for H2O2 (X1A) covering all dissociation channels
Abstract
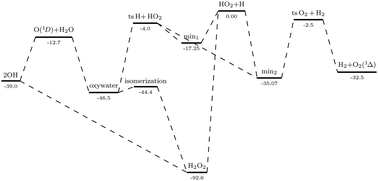
This work presents a new full dimensional potential energy surface for the ground singlet state of hydrogen peroxide, H2O2. This potential is based on a 3 × 3 matrix to accurately reproduce all the different dissociation channels in accordance with the Wigner–Witmer rules, namely, O(1D) + H2O(X1A1), OH(X2Π) + OH(X2Π), O2(a1Δg) + H2(X1Σg+) e H(2S) + HO2(X2A′′). It has been obtained by fitting more than 38 thousand ab initio energies computed using the aug-cc-pVTZ and aug-cc-pVQZ basis sets and extrapolated to the basis set limit. The functional form used to represent the four-body short-range interactions is based on a sum of polynomial functions of the fourth degree multiplied by a range factor, both built with intrinsic permutation symmetry and centred at specific reference geometries, to which the ab initio points computed are assigned based on a k-means algorithm. It also accounts for the electrostatic dipole–dipole interaction between two OH(2Π) fragments.


 Please wait while we load your content...
Please wait while we load your content...