Replica exchange molecular dynamics study of the truncated amyloid beta (11–40) trimer in solution†
Abstract
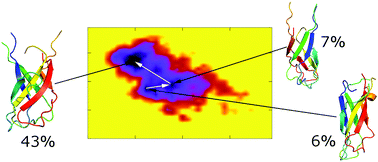
Amyloid beta (Aβ) oligomers are neurotoxic compounds that destroy the brain of Alzheimer's disease patients. Recent studies indicated that the trimer is one of the most cytotoxic forms of low molecular weight Aβ oligomers. As there was limited information about the structure of the Aβ trimer, either by experiment or by computation, we determined in this work the structure of the 3Aβ11–40 oligomer for the first time using the temperature replica exchange molecular dynamics simulations in the presence of an explicit solvent. More than 20.0 μs of MD simulations were performed. The probability of the β-content and random coil structure of the solvated trimer amounts to 42 ± 6 and 49 ± 7% which is in good agreement with experiments. Intermolecular interactions in central hydrophobic cores play a key role in stabilizing the oligomer. Intermolecular polar contacts between D23 and residues 24–29 replace the salt bridge D23–K28 to secure the loop region. The hydrophilic region of the N-terminus is maintained by the intermolecular polar crossing contacts H13A–Q15B and H13B–Q15C. The difference in the free energy of binding between the constituting monomers and the others amounts to −36 ± 8 kcal mol−1. The collision cross section of the representative structures of the trimer was computed to be 1330 ± 47 Å2, which is in good agreement with previous experiments.


 Please wait while we load your content...
Please wait while we load your content...