A metastable cubic phase of sodium niobate nanoparticles stabilized by chemically bonded solvent molecules†
Abstract
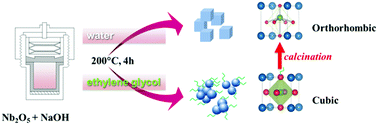
Structural modification, especially the stabilization of metastable phases at room temperature, has emerged as an effective strategy to understand their stabilization mechanism and improve their functional properties. In this work, a facile solvothermal approach is developed to synthesize metastable sodium niobate (NaNbO3) crystals with the cubic symmetry. XRD, Raman and TEM results all confirmed the selective synthesis of cubic and orthorhombic NaNbO3via adjustment of the reaction medium. The fact that traditional hydrothermal synthesis often yields orthorhombic NaNbO3 inspires us to elucidate the formation mechanism of cubic NaNbO3 with respect to the solvent effect. With the increasing post-calcination temperature, the as-synthesized cubic NaNbO3 gradually transforms into the orthorhombic structure, which is understood to be a recrystallization behavior, as evidenced by the XRD and TEM results. The organic molecules retained in the NaNbO3 nanocrystals, as suggested by UV-vis, FT-IR and TGA-MS results, have contributed to the stabilization of the metastable structure, demonstrated by the different temperature-induced phase transition behaviors in air and argon atmospheres, where the phase transition from cubic to orthorhombic would take place at a relatively higher temperature in argon. This work provides an alternative approach to synthesize cubic NaNbO3 nanocrystals, and the understanding of the stabilization mechanism could pave a new pathway for fabricating metastable materials.

 Please wait while we load your content...
Please wait while we load your content...