Understanding the role of hydrogen bonding in Brønsted acidic ionic liquid-catalyzed transesterification: a combined theoretical and experimental investigation†
Abstract
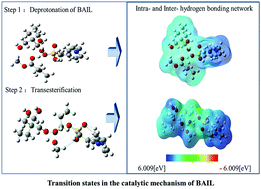
Brønsted acidic ionic liquids (BAILs) can play a dual role, as a solvent and as a catalyst, in many reactions. However, molecular details of the catalytic mechanism are poorly understood. We present here a density functional theory (DFT) study for the catalytic mechanism of the transesterification of methyl ester (ME) with trimethylolpropane (TMP), in the presence of three representative BAILs, namely, N-methylimidazole-IL, pyridinium-IL, and triethylamine-IL. The deprotonation of the BAIL cation and the transesterification step are investigated. Key inter- and intra-molecular hydrogen bonds (HBs) that govern the catalytic performance of BAILs were identified and analyzed using natural bond orbital (NBO) and atoms in molecule (AIM) methods. For the deprotonation of BAILs, it was found that the intermolecular O–H⋯O HB between the hydroxyl group of TMP and the oxygen of the sulfonic group of BAIL was indispensable for proton transfer. DFT computed free energy barriers for the transesterification step are in excellent agreement with the experimental results only after taking into account the BAIL cation–anion interaction in terms of HBs in which the O–H⋯O between the hydroxyl group of the anion and the oxygen of the sulfonic group of the cation was the strongest HB, suggesting the role of the anion in governing the catalytic activity of BAILs. The existence of the HBs suggested by DFT calculations was further validated using in situ FTIR experiments/ATR-FTIR.

 Please wait while we load your content...
Please wait while we load your content...