Electrochemistry of Fe3+/2+ at highly oriented pyrolytic graphite (HOPG) electrodes: kinetics, identification of major electroactive sites and time effects on the response†
Abstract
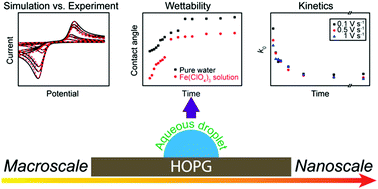
The electrochemistry of the Fe3+/2+ redox couple has been studied on highly oriented pyrolytic graphite (HOPG) samples that differ in step edge density by 2 orders of magnitude, to elucidate the effect of surface structure on the electron transfer (ET) kinetics. Macroscopic cyclic voltammetry measurements in a droplet-cell arrangement, highlight that the Fe3+/2+ process is characterised by slow ET kinetics on HOPG and that step edge coverage has little effect on the electrochemistry of Fe3+/2+. A standard heterogeneous ET rate constant of ∼5 × 10−5 cm s−1 for freshly cleaved HOPG was derived from simulation of the experimental results, which fell into the range of the values reported for metal electrodes, e.g. platinum and gold, despite the remarkable difference in density of electronic states (DOS) between HOPG and metal electrodes. This provides further evidence that outer-sphere redox processes on metal and sp2 carbon electrodes appear to be adiabatic. Complementary surface electroactivity mapping of HOPG, using scanning electrochemical cell microscopy, reveal the basal plane to be the predominant site for the Fe3+/2+ redox process. It is found that time after cleavage of the HOPG surface has an impact on the surface wettability (and surface contamination), as determined by contact angle measurements, and that this leads to a slow deterioration of the kinetics. These studies further confirm the importance of understanding and evaluating surface structure and history effects in HOPG electrochemistry, and how high resolution measurements, coupled with macroscopic studies provide a holistic view of electrochemical processes.


 Please wait while we load your content...
Please wait while we load your content...