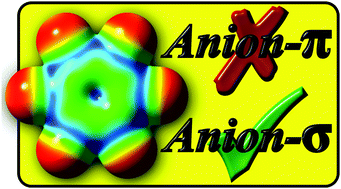
Should “anion–π interactions” be called “anion–σ interactions”? A revision of the origin of some hole-bonds and their nomenclature
Abstract
The so-called anion–π interactions are dissected to test if indeed the π system of the aromatic ring is the reason for the bond. In contrast, due to σ–holes the σ system is found to be responsible for a “pseudo-π hole” interaction. Models that form genuine anion–π bonds are discussed.


 Please wait while we load your content...
Please wait while we load your content...