Volume and structural relaxation in compressed sodium borate glass
Abstract
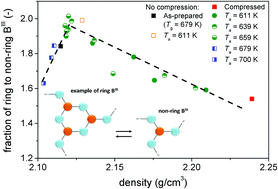
The structure and properties of glass can be modified through compression near the glass transition temperature (Tg), and such modified structure and properties can be maintained at ambient temperature and pressure. However, once the compressed glass undergoes annealing near Tg at ambient pressure, the modified structure and properties will relax. The challenging question is how the property relaxation is correlated with both the local and the medium-range structural relaxation. In this paper, we answer this question by studying the volume (density) and structural relaxation of a sodium borate glass that has first been pressure-quenched from its Tg at 1 GPa, and then annealed at ambient pressure under different temperature–time conditions. Using 11B MAS NMR and Raman spectroscopy, we find that the pressure-induced densification of the glass is accompanied by a conversion of six-membered rings into non-ring trigonal boron (BIII) units, i.e. a structural change in medium-range order, and an increase in the fraction of tetrahedral boron (BIV), i.e. a structural change in short-range order. These pressure-induced structural conversions are reversible during ambient pressure annealing near Tg, but exhibit a dependence on the annealing temperature, e.g. the ring/non-ring BIII ratio stabilizes at different values depending on the applied annealing temperature. We find that conversions between structural units cannot account for the pressure-induced densification, and instead we suggest the packing of structural units as the main densification mechanism.

 Please wait while we load your content...
Please wait while we load your content...