Self-assembly of partially fluorinated hexabenzocoronene derivatives in the solid state†
Abstract
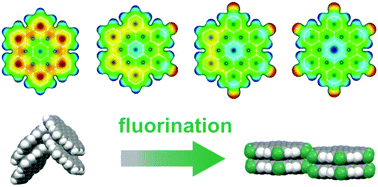
We report on the synthesis and structural characterization of novel, partially fluorinated hexabenzocoronene (HBC) derivatives. The fluorination of polycyclic aromatic hydrocarbons (PAHs) is a well-established method to enhance the stability of organic semiconductors (OSCs) and render them n-type. For HBC it has been observed that fluorination leads to a modification of the molecular packing motif from a herringbone arrangement to a parallel-packed motif. Here, we study whether this transformation of the molecular packing is also found for the partially fluorinated HBCs 2,5-difluoro-hexa-peri-hexabenzocoronene (F2HBC) and 2,5,8,11-tetrafluoro-peri-hexabenzocoronene (F4HBC). Combining powder diffraction and NEXAFS dichroism measurements, we reveal that indeed all partially fluorinated compounds adopt a parallel molecular packing, hence maximizing their intermolecular contact area. We identify fluorine–hydrogen bonds as the mediating driving force to specifically stabilize this molecular arrangement and direct self-assembly. Furthermore, we show that the relative orientation of the HBCs on the underlying surface can be precisely controlled by varying the substrate materials. Finally, the energetic states of the compounds are analyzed using photoelectron spectroscopy, optical spectroscopy and density functional theory to identify the effects of fluorination on these fundamental electronic characteristics.


 Please wait while we load your content...
Please wait while we load your content...