Ramachandran mapping of peptide conformation using a large database of computed Raman and Raman optical activity spectra†
Abstract
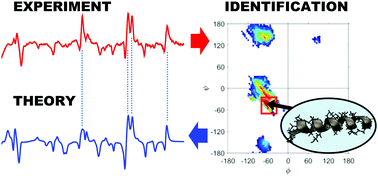
In the past few decades, Raman optical activity (ROA) spectroscopy has been shown to be very sensitive to the solution structure of peptides and proteins. A major and urgent challenge remains the need to make detailed assignments of experimental ROA patterns and relate those to the solution structure adopted by the protein. In the past few years, theoretical developments and implementations of ROA theory have made it possible to use quantum chemical methods to compute the ROA spectra of peptides. In this work, a large database of ROA spectra of peptide model structures describing the allowed backbone conformations of proteins was systematically calculated and used to make unprecedented detailed assignments of experimental ROA patterns to the conformational elements of the peptide in solution. By using a similarity index to compare an experimental spectrum to the database spectra (2902 theoretical spectra), the conformational preference of the peptide in solution can be assigned to a very specific region in the Ramachandran space. For six (poly)peptides this approach was validated and gives excellent agreement between experiment and theory. Additionally, hydrogen/deuterium exchanged structures and the conformational dependence of the amide modes in Raman spectra can be analysed using the new database. The excellent agreement between experiment and theory demonstrates the power of the newly developed database as a tool to study Raman and ROA patterns of peptides and proteins. The interpretation of experimental ROA patterns of different proteins published in the scientific literature is discussed based on the spectral trends observed in the database.


 Please wait while we load your content...
Please wait while we load your content...