A comparative study of nine berberine salts in the solid state: optimization of the photoluminescence and self-association properties through the choice of the anion†
Abstract
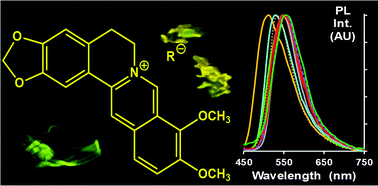
The arrangement of an ionic fluorophore in the crystalline state was regulated by the presence of various counter-ions and the effect on spectroscopic and self-association properties was studied. To do so, nine salts of berberine (i.e. a fluorescent natural alkaloid) were investigated. Most of them contained organic anions and were prepared using an ion-exchange process. Berberine chloride and hemisulfate were also used for the sake of comparison. The diffuse reflectance and photoluminescence spectra were recorded on powder compounds. All salts were emissive in the solid state and the emission efficiency was increased seven-fold with the nature of the anion. The optical properties were tentatively discussed on the basis of the crystal-packing mode. The possibility of implementing a bottom-up approach to generate microparticles was investigated using the reprecipitation method. Salts that contain the most hydrophobic anions gave a large number of homogeneous, elongated microparticles. This study showed that most of the berberine salts could be used as fluorescent materials, but proper choice of the anion allows using the self-association properties to best advantage.

 Please wait while we load your content...
Please wait while we load your content...