Initial steps for the thermal decomposition of alkaline-earth metal amidoboranes: a cluster approximation†
Abstract
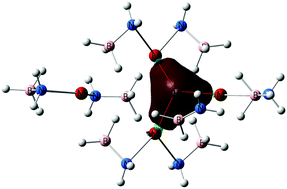
A DFT study of thermal decomposition mechanisms of [M(NH2BH3)2]4 clusters with M = Mg, Ca, and Sr is presented. Multi-step reaction pathways leading to elimination of the first H2 molecule are explored at the M06/TZVP level of theory. For all studied M, the clusters adopt similar structures and exhibit similar transformations along the reaction pathways. Their activation energies decrease in the order Mg < Ca ≤ Sr. Four metal atoms in the cluster form a rigid planar construction that is found to be nearly unchanged during all transformations. Cleavage of the B–H bond in the environment of alkaline-earth metal atoms leads to the “capture” of the released H atom by neighboring metal atoms with the formation of a M3H moiety. While the activation energies for the cleavage of Hδ− can be as low as 14.3, 22.6 and 23.3 kcal mol−1 for M = Mg, Ca and Sr, respectively, barriers for the subsequent cleavage of Hδ+via destruction of the M3H moiety are about twice larger.


 Please wait while we load your content...
Please wait while we load your content...