Plasma engineering of silicon quantum dots and their properties through energy deposition and chemistry†
Abstract
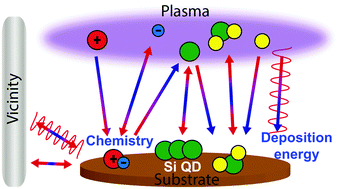
The characterization of plasma and atomic radical parameters along with the energy influx from plasma to the substrate during plasma enhanced chemical vapor deposition (PECVD) of Si quantum dot (QD) films is presented and discussed. In particular, relating to the Si QD process optimization and control of film growth, the necessity to control the deposition environment by inducing the effect of the energy of the key plasma species is realized. In this contribution, we report dual frequency PECVD processes for the low-temperature and high-rate deposition of Si QDs by chemistry and energy control of the key plasma species. The dual frequency plasmas can effectively produce a very high plasma density and atomic H and N densities, which are found to be crucial for the growth and nucleation of QDs. Apart from the study of plasma chemistry, the crucial role of the energy imparted due to these plasma activated species on the substrate is determined in light of QD formation. Various plasma diagnostics and film analysis methods are integrated to correlate the effect of plasma and energy flux on the properties of the deposited films prepared in the reactive mixtures of SiH4/NH3 at various pressures. The present results are highly relevant to the development of the next-generation plasma process for devices that rely on effective control of the QD size and film properties.

 Please wait while we load your content...
Please wait while we load your content...